The Past, Present and Future of Congenital Disorders of Glycosylation with Prof. Hudson Freeze
Episode Summary
Glycosylation, a biological process vital to life, is likely something you’ve never heard of before. It involves the addition of sugar molecules, known as glycans, to proteins and lipids which enable a number of crucial physiological functions, from immune regulation to cell-to-cell communication. This episode explores what happens when genetic mutations disrupt the glycosylation pathway as well as the detective work and collaboration required to diagnose and treat the resulting conditions. Hudson Freeze is the Director of the Sanford Children’s Health Research Centre and the Human Genetics Programme at Sanford Burnham Prebys Medical Discovery Institute, his research focuses on Congenital Disorders of Glycosylation, or CDG for short. Listen in as he reflects on the adventurous road to discovery of these rare conditions, the unlikely therapeutic options, the knowledge gap within the medical and scientific communities, and how a single discovery at Yellowstone National Park changed the course of history.
Conversation Timestamps
We Discuss:
- Hudson’s Background [02:11]
- Glycosylation vs. Glycation [03:37]
- Congenital Disorders of Glycosylation (CDG) [06:10]
- The Accelerating Pace of CDG Research [15:50]
- Hudson’s Groundbreaking Discovery [21:02]
- Utilising Animal Models for Glycosylation Research [25:36]
- Including Families and Patients in CDG Research [34:51]
- The Future of Diagnostic Options [40:55]
- The Role of Dietary Supplementation in CDG Treatment [45:37]
- Overcoming Funding Challenges [57:11]
About the Guest
Hudson Freeze
Professor Hudson Freeze is the Director of the Sanford Children’s Health Research Centre and the Human Genetics Programme at Sanford Burnham Prebys Medical Discovery Institute. Hudson is one of the leaders in research on glycosylation disorders, which are pathological conditions arising from errors in the attachment process of sugar chains, known as glycans, to proteins and lipids. These disorders are present at birth and can cause a wide range of symptoms, including developmental delay, intellectual disability, and other health problems. Hudson began his research on Congenital Disorders of Glycosylation (CDG) in the 1980s when the first CDG was identified. Over the decades, his lab was involved in the identification of a number of Congenital Disorders of Glycosylation. Today nearly 170 disorders have been identified. He is highly committed to searching for novel therapeutic and diagnostic options for patients with these rare conditions. Hudson previously served as the president of the Society for Glycobiology and the Federation of American Societies for Experimental Biology (FASEB). In these positions, he emphasised the importance of funding for basic research and encouraged scientists to actively discuss their work. At the beginning of his career, Hudson discovered a microorganism called Thermus aquaticus in the hot springs of Yellowstone National Park. Its thermostable DNA polymerase, Taq polymerase, became the foundation enzyme in the Polymerase Chain Reaction (PCR), the technique which revolutionised molecular biology. For this discovery, Hudson Freeze and his mentor Thomas Brock were awarded a Golden Goose Award in 2013 for groundbreaking, publicly funded research.
Conversation Highlights
"So glycosylation is the cellular metabolic process of adding sugar chains onto proteins or lipids. Glycation is strictly a chemical reaction where the different activated groups interact with each other and produce these things that, in the long run, are not good for you. And the advanced glycation end products are what happens when you have too much sugar, and that results in the complication of diabetes. So they're very, very separate. But scientists, physicians, Dr Oz writes in his book about the same sort of thing and mixes the two up. So it's very common. Let's get it right."
"And I picked up the tubes every day, and it had nothing growing. One day, I picked up the tube, and there was, and I thought, Oh my god, could this be something? So I real fast went over and took a sample and put it under the slide, and here are all these bacteria growing all over the place. And I mean, talking about it, even now, I still get goosebumps. Because I had seen something that nobody had ever seen in the world before, oh, this was so cool. And then, as time went on, exome sequencing wasn't developed. But the polymerase chain reaction, a key component of that was using an enzyme, the polymerase that we were able to see in Thermus aquaticus. And surprisingly, we didn't know this at the time, but in about 2013, the US Congress voted to be able to give an award called the Golden Goose Award to discoveries that appeared to have no use whatsoever as a waste of money. Why do you put money into this when you could be doing something important? And then discoveries like that, that then later went on to go, Oh, my God, you changed the world? Who would have guessed? So anyway, that was a proud moment."
"Most basic scientists are not intimately familiar with glycosylation. And I think the reason is that people may know biomedical scientists know that proteins exist, but they're much more focused on proteins and how DNA and RNA work and things like phosphorylation, where the tools are available to understand this better. You could take all the glycobiologists in the world and put them into a small gymnasium in Indiana, watching a high school game, you know, and that would be the entire field. And you look at neuroscience, there are 100,000 people. So familiarity with glycosylation and glycobiology and glycochemistry is not that common. And if you're training physicians, most of the time, basic scientists are participating in that. But if that knowledge isn't there, then the physicians who are being trained are not going to know about it. So there has been a big gap, an immense gap for what physicians will know. You have to be a highly specialised metabolic physician or geneticist to probably even have heard of this. And I've heard some tragic stories about physicians who weren't aware of these and could have put patients on therapy that would have made the total difference in their lives."
"So we had cells from a patient, we didn't know what the defect was. And we were using culture media, but we could see that there was a deficiency in glycosylation. And because most of these glycans are made from mannose, we just thought, well, maybe throw a little mannose, and maybe that would help, well the glycosylation got better. And so we actually went to the FDA in the US and said, Can we try mannose on ourselves? There was no literature at all on this. Can we try on ourselves this simple sugar? The FDA within three weeks responded and said, yes. So we lined up all the people that we had in the lab, and over two weekends, we dosed ourselves with varying amounts of mannose multiple rounds. And then, of course, we would tap our blood and watch the absorption and the clearance. So we had real data. And it was actually I guess, just a couple of weeks after that, a couple of days after that, I had a call from a physician in Germany, who says, we have a patient who has some sort of glycosylation disorder, we don't know what it is and we saw that you published this paper saying, maybe mannose could help. Do you have any idea how much mannose to give, how often? I said, funny, you should call, give this much, this often and the patient got a lot better."
Episode Transcript
Rina’s Intro
Rina Bogdanovic [00:05] Hello, hello, and welcome back to GlycanHub - the podcast in which we explore health, disease, and longevity through the lens of glycobiology. My name is Rina, and I am your host. In this episode, we will explore the role of glycans in normal physiological function and the consequences of glycosylation defects. As you might already know, glycosylation is a vital biological process that involves attaching sugar molecules, called glycans, to primarily proteins and lipids but also other molecules. What you might not know is that every single cell on Earth performs glycosylation. And these glycans significantly influence the structure, function, stability, and localisation of their target molecules. In earlier episodes, we have already explored their crucial roles in communication between cells and regulating the immune system. My guest today will introduce us to the consequences of genetic mutations which disrupt the glycosylation pathway through his work on congenital disorders of glycosylation, or CDG for short. We’ll discuss how different types of mutations impact the disease severity as well as the challenges and limitations of the current treatment options. Listen on to find out why he believes that mom power is the strongest force in the universe and how his chance discovery at age 20 contributed to the development of PCR, a technique that revolutionised molecular biology, enabling ground-breaking research and medical advancements. My guest today is the Director of the Sanford Children’s Health Research Centre and the Human Genetics Programme at Sanford Burnham Prebys Medical Discovery Institute. His research focuses on Congenital Disorders of Glycosylation, and he is highly committed to searching for novel therapeutic options for patients with these rare conditions. A warm welcome to Hudson Freeze.
Hudson Freeze [02:10] Thank you.
Hudson’s Background
Rina Bogdanovic [02:11] So before we start our conversation, I thought a good place to begin would be to ask you what your road to the field of glycobiology looked like. And particularly what is it that still keeps you interested in this field?
Hudson Freeze [02:23] Well, I think the thing that led me to it was my first introduction to it in my thesis work back in the early to mid-70s. And I thought this is something that nobody else is working on, maybe there is going to be an opportunity for somebody who goes a little off the beaten path, to do some things. And I think that I started there, some people came to me for analyses. And the analyses were about cells that couldn't do glycosylation correctly. And I thought - well, that's interesting. The other thing is that I have a disabled sister. And so when I heard that there were opportunities to actually work in a field that might have something to do with helping people with disabilities and their wellbeing, this, you know, earlier life got me ready for this. And the rewards that come from the patients and working with them, you can't beat that.
Rina Bogdanovic [03:34] And as our listeners will soon hear, you've continued to keep close contact with patients and their families through your research.
Hudson Freeze [03:36] Yes.
Glycosylation vs. Glycation
Rina Bogdanovic [03:37] Maybe the best place to make a clear definition at the beginning is just to define the difference between glycosylation and glycation. This is something we get asked very often. So I think it's maybe a good thing to distinguish at the beginning.
Hudson Freeze [03:55] Well, I think you've hit a very important point. In fact, there was a prominent article in science here just a couple of months ago. And it talks about all of the work that was done in looking for genes that might be important, as it turns out in ageing in males and females in different kinds of mice. And one of the genes they hit upon was a thing called DDOST. Well, that's a glycosylation enzyme. But the article later goes on to describe glycation. So they got it mixed up. So glycosylation is the cellular metabolic process of adding sugar chains onto proteins or lipids. Glycation is strictly a chemical reaction, where the different activated groups interact with each other and produce these things that in the long run are not good for you. And the advanced glycation end products are what happens when you have too much sugar and that results in the complication of diabetes. So they're very, very separate. But scientists, physicians, Dr Oz writes in his book about the same sort of thing and mixes the two up. So it's very common. Let's get it right.
Rina Bogdanovic [05:15] Okay, so just for our audience to be sure we are talking about glycosylation today. You just defined what it is. But could you explain how genetic mutations might affect it?
Hudson Freeze [05:26] Glycosylation is a very complicated pathway. And there's a whole series of pathways that require substrates, enzymes, and environmental conditions within the cell to make it work. So if any of those are disturbed, if any of them are disrupted, or mutations occur in any of those steps, you can have insufficient or incomplete glycosylation. And when that happens, there are a myriad of problems that happen in patients. And every cell carries out glycosylation, every cell on earth has glycosylation, so it's pretty fundamental to life.
Congenital Disorders of Glycosylation (CDG)
Rina Bogdanovic [06:10] And your work has very thoroughly focused on Congenital Disorders of Glycosylation. What are those?
Hudson Freeze [06:17] Well, those we define as mutations in any of the genes that affect the normal completion of sugar chains, we call them glycans, it's an easy way to refer to the overall group of molecules. So any mutation that interferes with the ability to complete those chains, put them in the right place, have them be functional, anything that does that, we would define that as a Congenital Disorder of Glycosylation. And, in fact, by looking at glycosylation, you can begin to define what those defects are. And we often, of course, by having patients with these, usually, they first went to their physician who more than likely doesn't know very much about glycosylation.
Rina Bogdanovic [07:10] And how common are these disorders?
Hudson Freeze [07:13] They're rare, I think that some of the most common ones, and there are only a few of those, would maybe have a total of a few 1000 patients. For the ones that are probably most popular and being worked on now. That one has about 1000 patients, but you know, there's no familiarity with it in all parts of the world. So I'm sure because it started in Europe, the US and Australia, New Zealand, that other portions of the globe are beginning to discover them. So that number is going to grow.
Rina Bogdanovic [07:51] And while we're still talking about mutations, could you distinguish between inherited and de novo mutations, and particularly how these might impact the severity and disease manifestation?
Hudson Freeze [08:05] There are, of course, inherited mutations that come from the individual parent, if it's an autosomal recessive disorder, as most of them are, then that requires one copy of the mutant gene from each of the parents, that's most common. There are a few that are dominant, meaning that only one copy has to be inherited. And some of those have very different phenotypes than the ones that are autosomal recessive. So de novo are spontaneous mutations that can occur in the embryo, they can occur in the parent’s germ cells, mother or father. And those mutations can then give rise to children that have abnormalities. And in fact, there are a few diseases recognised that are X-linked. So they're expressed primarily in males, unless it's lethal, then you never see it in males, because it's in females, because they have both X chromosomes. One is inactivated, but the bottom line is some of those de novo mutations can have very severe consequences. And, of course, the parents oftentimes are looking to themselves, you know, did I do something wrong? You know, is it me? And in many of these cases, of course, if they're de novo mutations, that's not the case. It just developed. It's very unfortunate.
Rina Bogdanovic [09:45] And I'm guessing depending on at which point in embryonic development these mutations occur, they might not be present in the entire body.
Hudson Freeze [09:54] Exactly, and that's what's important. There's a new disorder that we discovered four or five years ago, and now it's actually being found in adults who have seizures. And the gene is one that transports UDP-galactose from the cytoplasm into the Golgi. And so these patients, these adult patients would have seizures. And you know, they did exome sequencing, genome sequencing the whole body, and you couldn't find anything. And one of the standard surgical procedures, if you're not responding to medication, is to take out a small portion of the brain that's responsible for those seizures, and then look across that section and see if there are mutations that maybe occurred there. And in fact, a certain number of cases, maybe 10% of some of the cases, have those kinds of seizures that can be caused by a single mutation in that gene that is localised to that part of the brain. You would have never guessed. But that's the sort of the next stage I guess, of what we can do with genomics and understanding how these things work. So now it turns it back over to the fundamental glycobiologist to say, Well, now let's take a look. What do we know about those cells that are affected? What different kinds of glycans? How are they impacted? So it gives a lot of opportunity for, I guess, you can say crossfading, clinical science informs basic science, basic science informs clinical science. We are all in the ecosystem.
Rina Bogdanovic [11:42] When it comes to, again, genetic mutations, is it usually common that it's a monogenic disease? Or is it polygenic meaning multiple mutations contribute?
Hudson Freeze [11:52] That's again, another interesting question. Most of the ones that we look at are monogenic, and they're easier to track, easier to trace and understand. But there are some where there are contributions from different heterozygous mutations in the same individual that impact different aspects of the same pathway. For instance, there was a paper a few years back that showed there were individuals who got deep vein thrombosis, and that's forming clots, well, the only time they would get that is if they were drinking heavily. While we know that heavy drinking can impact glycosylation, and it turns out that some of those people had mutations in the gene that is responsible for one of the major causes of a Congenital Disorder of Glycosylation called PMM2. And so I'm heterozygous for that, that most common mutation as well. And so I have a lot in common with the patients that I work with. So we're, we're kinshipped. But these people under the stress of extra alcohol, and the stress of not having quite enough enzymatic activity, they would not form clots. And the reason was that most of the clotting factors, essentially all of them, are glycosylated. So if you're not sufficiently glycosylating, then you may be deficient in those clotting factors.
Rina Bogdanovic [13:31] And so if it wasn't for that behaviour of drinking heavily, they would have never known.
Hudson Freeze [13:35] Yeah, that's right. So thank you, folks, for informing the basic science and letting us know.
Rina Bogdanovic [13:41] But as you said, glycosylation happens all over the body, in every cell of our body. And so, how might that impact the different symptoms experienced by patients with CDG?
Hudson Freeze [13:53] Because it happens in different parts of the body, one of the most common forms, and I guess even the most common symptoms are intellectual disability, seizures, and growth retardation. And so in those cases, you know, of course, we know that glycosylation is really important in the structural formation of the brain, but also in being able to learn to have normal neurotransmission. And so those are quite common. And I think you can find patients who have other kinds of disorders because now there are almost 170 different kinds of CDG. So with that large, large number, you can imagine that there is a whole spectrum of different impacts. And there's one disease, for instance, that we study that doesn't show any intellectual disability, but what it does, is result in what's called primordial dwarfism, which is the little people. And the little people actually have normal intellectual development. But they're short, they have short arms and legs, and rather short fingers, and a very characteristic facial appearance. So that is caused by a mostly de novo mutation in one copy of one gene, that we know when you have two copies, that gives you a very different and much more severe type of CDG. So depending upon the load that the individual type of cells or the organs have to deal with, that will sometimes determine what the outcome is.
The Accelerating Pace of CDG Research
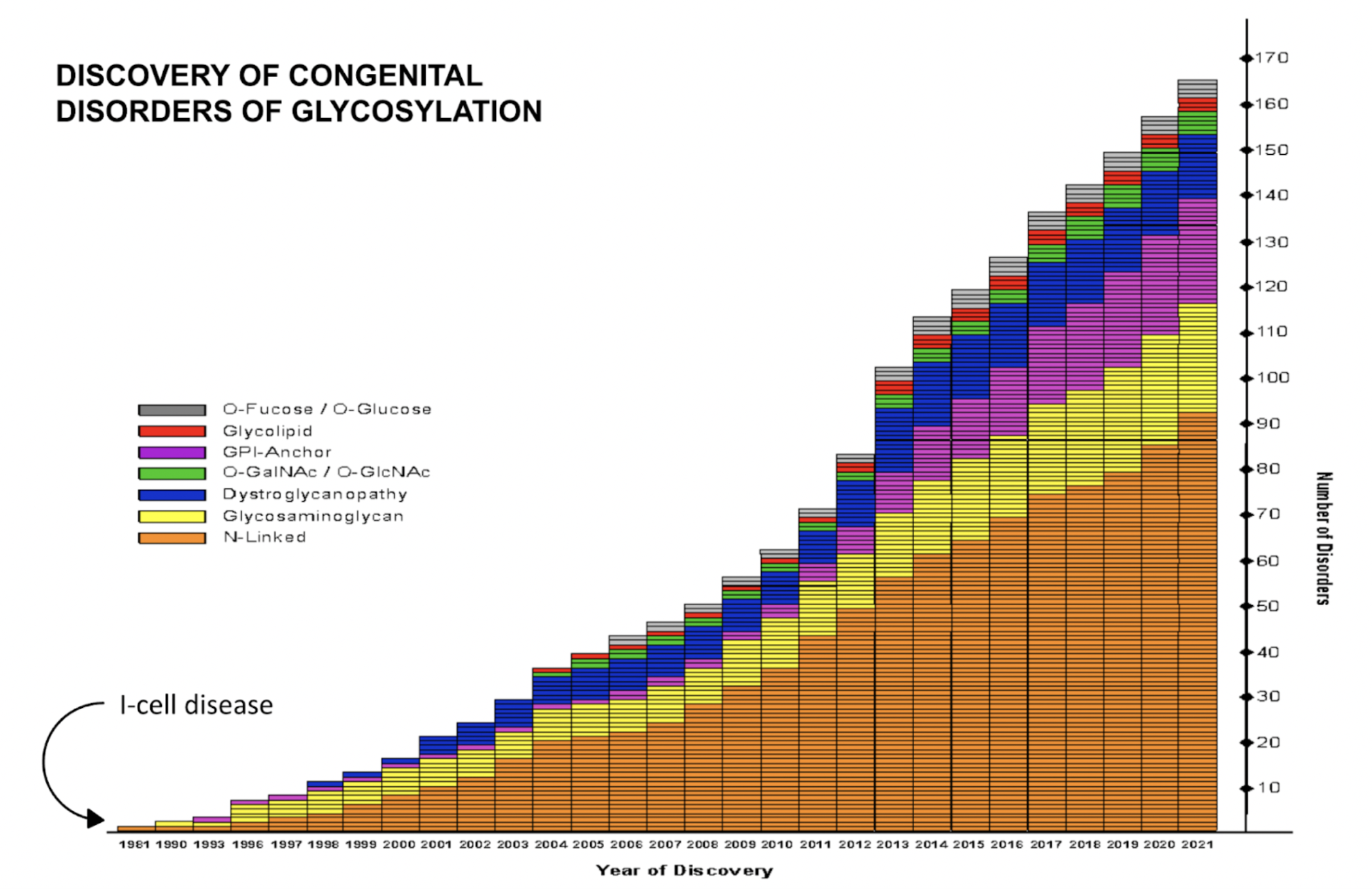
Rina Bogdanovic [15:50] And you mentioned, if I'm not mistaken, there are currently 170 CDGs.
Hudson Freeze [15:54] Almost.
Rina Bogdanovic [15:56] Almost 170. Just so that our audience can appreciate the speed of development in this area, how many CDGs were identified when you first started working in this field?
Hudson Freeze [16:12] One. And that was a disorder called I-cell disease. And I-cell disease was considered a lysosomal storage disorder. And the reason was that you accumulated material in the cells, this is usually lethal by about seven or eight years old. But the reason that you accumulated all of these things within cells was that a molecule called mannose-6-phosphate couldn't be added to a sugar chain. And that was important in targeting lysosomal enzymes very specifically, to the lysosome. And if they don't go to the lysosome, of course, they can't do their work of digesting the material in the cell, and they were secreted. So it was a secretion effect. But in fact, even though it resulted in the accumulation of things within cells, that was a secondary effect, the primary effect was not putting on mannose-6-phosphate. So one disorder was known. And I think we've been involved, either directly or in collaboration with other labs with the discovery of about 25 or 26 disorders. And I was working in I-cell. At the time it was being worked out, and my sort of original work, believe it or not, in slime moulds identified some mannose-6-phosphate there. So it was a natural evolution, to go from there to working with these rare disorders and with patients.
Rina Bogdanovic [17:52] It’s funny how we have stuff in common with slime moulds.
Hudson Freeze [17:55] Isn’t it?
Rina Bogdanovic [17:58] You mentioned exome sequencing. And now focusing a little bit on research developments in this area, how has exome sequencing contributed to the development in this area, and it also may be good to define what exome sequencing is.
Hudson Freeze [18:12] There is the whole genome I think that people will be familiar with. And that genome actually has about 98% of the DNA that's never made into proteins. That portion that is made into proteins is thought to be the activity in functional aspects, although the rest of it is very important as well. That's from a series of exons. So there are introns and exons. Okay, so the introns are not part of the finished protein. But the exons, those are made into proteins, that's only 2% of the genome. So when people look at exome sequencing, they're only looking at 2%. But most, but not all of the diseases come from mutations in that portion. Although the other parts, the introns, you know, will also sometimes have an effect. But exome sequencing has been an absolute breakthrough. I mean, I look at the growth of these disorders, from when we first discovered them. And then it was sort of using bootstrap biochemistry to figure out what the genes were and then went back to show that there were mutations in those genes. Now with exome sequencing, it's reversed. So you have these really wonderful and now fairly inexpensive ways to be able to see different mutations and with computational biology, you can figure out which mutations are likely but not guaranteed to be the cause. But with that, you can now go back and look at the glycosylation in the cells or a biomarker that comes from the patients. And you can be able to say yes, it's very likely that those mutations are causing this problem. That's been a tremendous breakthrough.
Rina Bogdanovic [20:18] And do you have any information on how many disorders have been discovered or identified since exome sequencing began to be used?
Hudson Freeze [20:27] You know, I can probably get some reasonable idea of this because I have a graph that I actually had put together. And if you say that, let's say the exome was done, oh, I don't know in 2001, 2002. But it was fairly expensive, so not everybody had it. At that point, we knew all about maybe 25 disorders. Now we're up to 165 or more. So without exome sequencing, we'd still be scrounging around, and would not have discovered these things.
Hudson’s Groundbreaking Discovery
Rina Bogdanovic [21:02] Something I found very interesting about you when I was reading a bit about you was your contribution to the development of PCR. Now, I think most people have probably heard about Polymerase Chain Reaction following COVID. We've all had our tests. But otherwise, it's also used in exome sequencing. And your contribution was through the discovery of a microorganism called Thermus aquaticus, from the springs in Yellowstone National Park, if I'm not wrong. And so what was it that you were investigating at the time?
Hudson Freeze [21:36] Basic science. And again, the solution to medicine is going to be found in basic science. So at the time, I was a 20-year-old student, who had never been out of the state of Indiana, to speak of. And my mentor, Tom Brock, said, Well, look, we're going to Yellowstone this summer. And I said, Why are you going to do that? He says, Well, you know, there are these hot springs in Yellowstone. And we want to see if there are bacteria that can grow there. I said, Yeah, that sounds really nice. In part, because my high school science project was about life on Mars. So I was accustomed to already thinking about extreme environments, right? And so here was something that was at the other end of the spectrum from cold to almost boiling hot. So I was there for about five weeks in Yellowstone, working with, going out in the field, collecting samples, doing all the basic research, because we had no idea that this would lead anywhere. It was fundamental science, it was funded by the National Science Foundation, which said, Well, yeah, it's kind of interesting. Go take a look at that.
 (Image credit: Getty)
(Image credit: Getty)
And so I used as my senior research project, the discovery of these organisms, and it was only about a month later, I had samples back in the lab. And we incubated some of these from samples from different springs, and we incubated them at about 70, 72 degrees Centigrade. And I picked up the tubes every day and it had nothing growing. One day, I picked up the tube and there was, and I thought, Oh my god, could this be something? So I real fast went over and took a sample and put it under the slide and here are all these bacteria growing all over the place. And I mean, talking about it, even now, I still get goosebumps. I mean.
Rina Bogdanovic [23:41] No, I bet.
Hudson Freeze [23:43] Because I had seen something that nobody had ever seen in the world before. Oh, this was so cool. So at the time, you don't have an application. And then as time went on, exome sequencing wasn't developed. But the polymerase chain reaction, a key component of that was using an enzyme, the polymerase that we were able to see in Thermus aquaticus. And, surprisingly, we didn't know this at the time, but in about 2013, the US Congress voted to be able to give an award called the Golden Goose Award to discoveries that appeared to have no use whatsoever as a waste of money. Why do you put money into this when you could be doing something important? And then discoveries like that, that then later went on to go Oh, my God, you changed the world? Who would have guessed? So anyway, that was a proud moment.
Rina Bogdanovic [24:57] I bet, it's why I decided to ask you about this. It's crazy. So you went on an expedition, not having any idea if you were going to find something. You found the bacteria, and later on, an enzyme was taken from the bacteria and has been incorporated into a reaction that’s now modernised medicine and basic research. It's crazy.
Hudson Freeze [25:17] I would say it actually created the biotech industry. I can guarantee you there's not a company on the face of the earth that's doing biotechnology, that doesn't use PCR. So whether this is hundreds of billions or trillions, I don't know, I guess we have to see about the next pandemic.
Utilising Animal Models for Glycosylation Research
Rina Bogdanovic [25:36] Coming back to Congenital Disorders of Glycosylation. I'm curious, are animal models used in research? And how accurately can they represent human conditions?
Hudson Freeze [25:48] Animal models are really essential. And you know, especially if you find a drug and use the drug, you have to test those in animals. So you need an animal model to be able to show that you have efficacy before you can ever take something like this into humans. So that's going to be at the far end. But if we take it from a research standpoint, we want to be able to make more discoveries. And the best way to do that is to use cellular or organism models of the disorder. And in many cases, those have actually revealed a lot of things that we hadn't expected. So they're absolutely essential. And I know that we have done experiments in mice, many people also do their experiments in mice. But there are also other systems that are used. And that includes flies like Drosophila, worms like C. elegans, and yeast. So oftentimes, it's easiest to work on yeast because they grow fast, you can do the genetics easily, and you can find a phenotype. The problem can be that that phenotype is limited to what the yeast is going to do, you know, make bread and beer and grow itself. So you need something that is going to mimic human physiology. And I'll give another example of something that we did. We made a mouse model, of one of the diseases that we discovered. And one way we made it, it didn't have any phenotype that we could see, we expected to see certain things going on, but it didn't happen. It's only when we stressed the animal that now phenotypes appeared. And so that was really important, because it said, here is what you need to have a human habit disorder. Maybe you need much, much less before you see it happening in the animal system. So making those transitions can sometimes be difficult, but I can tell you, some really important breakthroughs have come from looking at simple model organisms. And we can talk more about specific therapies if you want, but they're absolutely essential.
Rina Bogdanovic [28:17] Before we go into therapies, something I'm going to talk about is education in the medical system. Now, we said the number of CDGs has increased very rapidly in the last few decades. And so imagine there has been a pretty big educational gap concerning the education of clinicians, had these discoveries been adequately incorporated into medical syllabi?
Hudson Freeze [28:41] No. In a word. And I think it's important to point this out. Most basic scientists are not intimately familiar with glycosylation. And I think the reason is that people may know, biomedical scientists know that proteins exist, but they're much more focused on proteins and how DNA and RNA work and things like phosphorylation, where the tools are available to understand this better. You could take all the glycobiologists in the world and put them into a small gymnasium in Indiana, watching a high school game, you know, and that would be the entire field. And you look at neuroscience, there are 100,000 people. So familiarity with glycosylation and glycobiology and glycochemistry is not that common. And if you're training physicians, most of the time basic scientists are participating in that. But if that knowledge isn't there, then the physicians who are being trained are not going to know about it. So there has been a big gap, an immense gap for what physicians will know. You have to be a highly specialised metabolic physician or geneticist to probably even have heard of this. And I've heard some tragic stories about physicians who weren't aware of these and could have put patients on therapy that would have made the total difference in their lives. And, you know, you can't ask every doc to be familiar with every disorder. And I get that. But we do need to get out there more, we do need to talk more, and we need to integrate this into undergraduate and graduate courses. I just gave a lecture on glycobiology, and CDG in the fundamental biomedical course. And there are a couple of things that help out. One was when Harrison Ford made the movie called Extraordinary Measures. And he gets to use the line in there, he says - What’s the matter, Sal? Not up on your glycobiology. So I use that and I mean, that's a bit of a hook to bring people in. It's, well, what's this guy gonna talk about? And then he has another line, he says mannose-6-phosphates, you know, and again, that works for being able to do the things that we're talking about. That helps. And I think another additional boost is going to come out. Because Carolyn Bertozzi this year won the Nobel Prize for applying real clever chemistry, to glycome, to cells, to organisms. So I think these techniques are now going to elevate the awareness of glycosylation. And Carolyn has been doing this for a long time. She's absolutely brilliant. And she's made so many contributions to the field. So there is a gap, but we're trying to close it.
Rina Bogdanovic [32:02] For our audience, who might be composed somewhat of clinicians who are interested in finding ways of perhaps educating themselves more on this. Are there any resources you might want to point out?
Hudson Freeze [32:14] Sure, there are a couple of family groups. Now the family support groups have really grown in the last, I would say, 10 years. And it is really quite impressive, because there is, how can I say this, no force stronger than mom power. And so you have a lot of mothers, mothers and fathers, whole families who have organised to get behind, I'm going to take care of my child. And so they form these individual groups. So, you know, one of them is called CDG Care. That's resources for the patients whose doctors happened to find something, they can now take that and say, Here you go, look at this. And the doc can get educated as well. Now, there's also a consortium that has been pulled together an NIH-funded consortium in the US, it's called The Frontiers of Congenital Disorders of Glycosylation Consortium. And that is a consortium of about15 different sites, and their primary clinical sites in the US, but we're also expanding those into areas in Europe and beyond. And so the idea there is we're going to collect a natural history of different disorders, enrol the patients so that they can have annual checkups, or semi-annual checkups. And then we'll have the material because we're also looking at biomarkers to be able to stage these. And I can tell you now, that's really pretty amazing. Two to three years ago, there were no therapies out there. But now there are about five different therapies that are at the point of entering or almost getting ready to enter clinical trials as a treatment for some of these disorders. And if you go to the FCDGC, you'll find those resources there. And if you're a physician, and you think you want to join this group, you want to become active, you're welcome. And there's going to be a lot of welcoming people, families, and clinicians, this is all based at the Mayo Clinic in Rochester, but as I say there are sites all over the country and expanding worldwide.
Including Families and Patients in CDG Research
Rina Bogdanovic [34:51] Excellent, thank you. And how did families and patients with CDG, how did they get involved in your research? How has that been organised?
Hudson Freeze [35:01] Well, you know, in the earlier days, let's say, you know, in the time when there's only 20 to 30, disorders, parents, again - mom power, would hear something about glycosylation. If they had some evidence of something being wrong, they would go to the internet, and they would find me. You know, I still quite frequently get calls and emails from families saying, Can you help us? Can you just tell us anything? And then sometimes what I would do is say - well, I need to talk to your physician, because that's the primary relationship. And those physicians would then loop me in, and we would get a functioning ecosystem, where we might be able to advise the physician about what to do, and the samples that we might need. And so that was our primary connection. Sometimes, of course, it would be the other way around. A physician would be contacting us to say - I have a patient, what can you do? What resources can you give me? Can we be sure that this is actually a disorder of glycosylation? And we've done that in many, many instances. And, you know, I think our basic science papers now, are maybe even fewer than our clinically oriented ones, because I mean, I view our job as being the interface, right, being able to link the communities together. And that's what's important because come on, this is about the kids. And the adults that have CDG.
Rina Bogdanovic [36:44] So your institute has organised these patient days every year where you bring families with patients, you bring clinicians, bring scientists, and allow them all to interact and meet each other. What has been the result of these meetings?
Hudson Freeze [36:59] Outstanding. I know that there are scientists that I've invited whose research might be peripherally involved in CDG, but that they can make a contribution. And so when they come in, sometimes they're a little shy, they’ve never met a family before, and they're sitting in the midst of the families, they're seeing the kids playing on the floor. And, some of them get a little bit choked up. And they'll tell me afterwards, you know, this is the best meeting I've ever been to, I mean, this really changes my life in the lab. Because I see what we can do. You know, sometimes they need a refresher course to do that. And the families benefit because they may get some time with their individual clinicians, and get to know them as people. And sometimes physicians get to know the patients in a different way. We have one patient here in San Diego, I just love this guy. His mom describes him as 7 going on 34. And he wasn't diagnosed till a couple of years ago. And he is just charming. And you can tell he's got a disability, but he can speak, he can walk, he has problems, but he can play the drums. And he does a marvellous job of playing the drums and I got a video of his kid, you know, playing the drums with a blues band. And there's another patient that I absolutely love. She is an equestrian. She does dressage in Australia. And she came to one of these meetings, and we were able to have her hook up and go to the stables of one of her heroes who had been in the Olympics. And he got to take her around his stables, she got to ride on the horse. And then later because she was part of the meeting, she got to demonstrate that she does - dance. She dances even though she's in a wheelchair, she will be on the ground and she will make these beautiful motions, and then in the wheelchair and moving around. And she insisted that I sing a song with her. So again, you know, these are the things that link people together that take us out of where we normally would be and say - well, we're part of this ecosystem. We're all together. So families love it, physicians love it. Scientists love it. We do it every couple of years.

Rina Bogdanovic [39:50] Sounds really wonderful. And do people from all over the world come to this meeting? Is it international?
Hudson Freeze [39:56] Yes, there are but I have to say that there is also another international meeting that is coming up in the near future. And that is being organised in Europe. It's called the Congenital Disorders of Glycosylation World Conference. Now it's primarily centred in Europe. But people from the US and Australia, and I'm sure some of the other areas, which is the Middle East are also coming. Now that meeting is going to occur from July 21 to July 23, of this coming year. And that's in Lisbon. And I can send you a link for that so that if people want to get more information, they can look it up on the web.
Rina Bogdanovic [40:43] Excellent, I’ll put it in the description of the episode.
Hudson Freeze [40:50] All right, sounds good.
The Future of Diagnostic Options
Rina Bogdanovic [40:55] Some of your work is also focusing on the development of new molecular diagnostic methods. How do you aim to improve the current diagnostic options? And what are some of the challenges that need to be overcome?
Hudson Freeze [41:00] Well, I think there are some disorders that are still not easy to diagnose. And some of those are because once again, they may not be well known, but there are a considerable number of these. These ones are called GPI anchor deficiencies. And so there are people now at the Mayo Clinic, who are actually working on some new methods using it's called CODEX, which is a way of being able to tag many, many proteins with heavy isotopes. And then by mass spectrometry, look at that. So that is going to be useful. It's very early stages of development, but you can then begin by using just a blood sample to see in those cells, what proteins are missing, and what proteins are not. And maybe you even begin to sort out different groups with different types of disorders. That's one thing. The other thing that is really quite exciting is that there is the repurposing of a drug Epalrestat which is licensed in Japan and is for treating diabetes. And one of the basic scientists Ethan Perlstein did a screen looking at approved drugs and asking which one might be able to improve a worm model, C. elegans, model of this major disorder. And he found one and this was the Epalrestat. The Epalrestat turned out to not only improve the biochemical features but it also in n=one patient, you got to start somewhere, improved her condition. So now there are clinical trials that are just starting, where patients with this disorder, it's called PMM2-CDG will be able to take this drug and be monitored for their improvement. And it looks very promising. Now one of the things that were interesting is that Epalrestat is the aldose reductase inhibitor. And what it does, is it prevents sugars from being turned into sugar alcohols, it turns out, that these CDG patients that are part of this clinical trial have elevated levels of sorbitol, which is the reduced form of glucose and of mannitol. And when you put the patient on this, you can actually reduce the amount of those back to normal levels. So you can take a standard assay that was used for monitoring diabetes sorbitol, and you can now use it for CDG. And using things like this is a real godsend. Because now we can take things we know and apply them, we can take new things that we're developing and apply them. So again, it's the ecosystem. It's the balance between basic science, biomarkers, model organisms, and the patients with the docs linking us all together.
Rina Bogdanovic [44:28] Now I especially like the idea of being able to repurpose something that was developed for an entirely different purpose on a disease that they probably didn't even know about when they were developing the medication.
Hudson Freeze [44:39] No, nobody would have cared because I can probably with some confidence, say that most people in glycoscience, never think about sugar alcohols. Well, we should. But then again, you know, coming from something better. If it's a patient, you take it back and say, well, we got to rethink microbiology, and how different molecules are being processed within the cell, the stuff that we never really cared about, maybe that's influencing. And we know what's influencing glycosylation. So there's all this framework of metabolic things going on inside the cell. You know, they're not just a big mixture of things. But maybe they're actually looking more like the Tokyo or the DC subway, where they're all these interconnections and all these pathways, we've never defined them. But they're important.
The Role of Dietary Supplementation in CDG Treatment
Rina Bogdanovic [45:37] Now one treatment option that I found very interesting was the application of dietary supplementation in these conditions. And so where is this type of treatment applicable?
Hudson Freeze [45:49] I think it's applicable to a limited number of diseases. The sugar supplements themselves are sugars, you can honestly buy them on Amazon. But most of them have, well, essentially, all of them have not been through clinical trials. So if there is a little bit of literature out there, that shows maybe on even a couple of patients or just even themselves, that there can be improvement, then families will go to Amazon or wherever and do this themselves. We try to encourage people not to do it because then you can never get the real data that will allow you to then say this is how much, this is how frequently. And so we first came up with the idea of using mannose as a treatment. And it takes care of most but not all symptoms of a deficiency called MPI-CDG. And MPI-DG is caused by a deficiency in believe it or not making mannose-6-phosphate and converting fructose-6-phosphate to mannose-6-phosphate. But if you provide mannose, then mannose just runs right around that block and says we don't care and you supplement. So that's important to be able to do that. And you say, well, there's probably no side effects. Well, we found out again, using a mouse model, that if you give a mom who would be a carrier of this disorder if you give her mannose, this in the embryo can result in blindness. And we would have never predicted this blindness in the embryo. The lens doesn't develop now, is that going to happen in humans? Well, we don't have an idea. But as soon as you find out, you go, Oh, my God, we've got to be careful with this stuff. And we saw that we explained the basic science behind it, so that's not a reason not to use it, but it is a reason that you have to have clinical trials. There's also galactose, which is one of the components and one of the disaccharides or monosaccharides of lactose, and that can be used to treat several of these disorders. Fucose is another one. And you can use fucose to actually improve the conditions of some of these patients, you know, maybe not all of them. But again, we have to know and understand some of the basic science, but originally on the basic science, it shouldn't have worked. Because fucose was thought to be not metabolised, you know, maybe a little contribution. So fucosulation, but in turn, it turns out that it can be a major contribution, if you have extra supplements, like fucose and so all of this stuff was unknown. Now we're beginning to understand it. And again, it takes you back to the basic science. And there's one other component that is being used, not only in CDG-related disorders, but even there are ideas that this may be important in rather common disorders. And that's the use of N-Acetylglucosamine. That is getting some evidence that it may be helpful in the diseases slipping my mind at the moment. But it's I'll figure it out. But there's evidence that it is improving some of the symptoms. So you know, I think that sugar supplements are not normally thought of as medicines. We do need to validate them. But it's important and it can be used, you need to do it carefully. If you want to know what carefully means - contact the consortium that we have for CDG. That will give you a better insight.
Rina Bogdanovic [50:19] How long ago have you been looking into, or in general, have people been looking into the potential supplementation of these sugars? For people with CDG?
Hudson Freeze [50:30] We started it with mannose back in about ‘96. Do you want the story on that?
Rina Bogdanovic [50:33] Sure, sure.
Hudson Freeze [50:35] So we had cells from a patient, we didn't know what the defect was. And we were using culture media, but we could see that there was a deficiency in glycosylation. And because most of these glycans are made from mannose, we just thought, well, maybe throw a little mannose, and maybe that would help, well the glycosylation got better. And so we actually went to the FDA in the US and said, Can we try mannose on ourselves? There was no literature at all on this. Can we try on ourselves this simple sugar? The FDA within three weeks responded and said, yes. So we lined up all the people that we had in the lab, and over two weekends, we dosed ourselves with varying amounts of mannose multiple rounds. And then, of course, we would tap our blood and watch the absorption and the clearance. So we had real data. And it was actually I guess, just a couple of weeks after that, a couple of days after that, I had a call from a physician in Germany, who says, we have a patient who has some sort of glycosylation disorder, we don't know what it is and we saw that you published this paper saying, maybe mannose could help. Do you have any idea how much mannose to give, how often? I said, funny, you should call, give this much, this often and the patient got a lot better. And then we realised that, in fact, all the biochemical parameters, his growth, and all the other problems had improved based on the mannose. And so that was our first clue. Back then, that, you know, this is something that deserves exploration. And then that led to glucose, and then to galactose. And now N-Acetylglucosamine. I mean, so it was sort of another one of those - yeah, it's basic sciences. Take a look. But there's a lot more to the story.
Rina Bogdanovic [52:52] You said several different treatments are being developed. Could you elaborate a bit about those?
Hudson Freeze [52:59] Galactose is being used for a disorder called PGM-1, which is a deficiency in Phosphoglucomutase 1. That deficiency has a series of symptoms, and there's evidence that that [galactose] is improving the phenotype of these patients. There's very good evidence for that now, so that is about to enter clinical trials. Galactose can also be used for galactosylation deficiency, and that is in this carrier of UDP-galactose into the Golgi. There's evidence that giving some galactose can help in a deficiency called LED II - leukocyte adhesion deficiency type two, that fucose can help some of those patients. And, again, it was not thought that this would make any difference, but in fact, it does, because they usually get an immense amount of infections, they can't have their white blood cells extravasate to fight the infection in the tissues, it stays in the circulation. And the reason it does is that there are fucosylated glycans, which are necessary to allow the first step before the white cells get into the tissue. And that step is blocked once you provide fucose, within a couple of days, the sugar chain is made in the white cells that can go in and fight infections. So that's another one. N-Acetylglucosamine is a potential therapy for a disorder called NGLY1 deficiency. And I have to say that it was a parent who figured this out. And the way it worked was this parent very, very bright, very loving parents noticed that or thought that maybe there wasn't enough N-Acetylglucosamine for many reasons. And so they decided to dose their son with it. He had, he couldn't form tears. And you get these crusty things on his eyes, you know, and they were at one point, they're saying - How can we take care of our kid? And they've put eyedrops in to moisten, you know, the tears because he wasn't producing, they gave him N-Acetylglucosamine. The crust went away. He was starting to make a little bit of tears. They took him off because they came to me and said - What should we do? I said, well, sorry, but you got to take him off for now, and the crust re-appeared. So we put him back on it. And at that time, the patient's grandmother came to be with the boy because the parents were going to be gone. The grandmother said, I have never seen his eyes look so good. She didn't know about N-Acetylglucosamine. By the time the parents came back, and he wasn't getting any dosing, it had gotten worse. And she says, oh I must be a terrible grandmother, you know, he's getting worse, right in front of my eyes. So there, the experiment was done. And now there are a number of children who have taken this. So, N-Acetylglucosamine is going to enter into a clinical trial, again, you know, with all the right kinds of measurements probably within the next year. So it's these sorts of things where, you know, you're bootstrapping, you're again in this ecosystem, and everybody's making a contribution.
Rina Bogdanovic [57:00] It's absolutely amazing. Just what understanding of basic science can get you. It's real detective work.
Hudson Freeze [57:06] Yeah. It's a lot of fun, I gotta say.
Overcoming Funding Challenges
Rina Bogdanovic [57:11] Now, one thing I want us to consider before we conclude our conversation is funding. These are very rare conditions. And so could you comment on the challenges of finding research funding for these disorders?
Hudson Freeze [57:25] Sometimes it can be very difficult. And we've been fortunate in getting funding from the NIH. And I think at least part of that was showing that there was a relationship to a disorder, but also bringing in the fundamental science. So the NIH loves fundamental science, especially when it applies to patients. Now, there probably is a, what can I say? A breakeven point where some reviewers or maybe even some people in the NIH administration would say, well, that's just too rare. I hope that's not the case. And if the opportunity to make contributions and understand basic science and affect even a small number of patients can be done, the right group of reviewers can look at that and say - this is really important. I have also, because of my familiarity with that system, realised that there are not all that many people trained in glycobiology like chemistry, who can appreciate that connection. So if those people are not on the review panels, they don't know, don't think this stuff is important. I mean, I study phosphorylation, or I study other things, I can't see that that's important. You need people on those review panels who know glycosylation and not every glycosylation grant is going to be up at the top, you can stand improvement. But that's one of the things that we need to do. And that's where education is really important. The other aspect, I have to say, and we've been extremely lucky in this is that sometimes there are parents and families of patients that we've worked with, and sometimes they have been so incredibly generous to the lab because they realise our connection, and they want to support us. And there have been literally hundreds of donations to the lab. You know, some from I don't need birthday presents redirected to our lab. Another person might hold a fundraiser in a bar. And then, you know, provide us with the money from that, somebody ran the Grand Canyon as a marathon and said you know, here’s money from that. And, there were others that were $10 and $20 donations, you know, some of my family might send something. And I donate, you know, it's, if your heart's in it, you're connected. That helps, but we need the big 100.000s in education to take care of that.
Rina Bogdanovic [01:00:50] And especially when it comes to scientific advocacy. Could you tell me a bit about FASEB?
Hudson Freeze [01:00:56] Yeah, FASEB in the US is an organisation of over 30 Biomedical Research societies, genetics, physiology, glycobiology, and biochemistry, and they have a special place in advocacy. Because we, as scientists, can go to Capitol Hill sponsored by FASEB and organised and the people that do this, have credibility with the staff, people, and in Congress, and they can set up meetings for us. And we can go tell them the stories about what is going on, what's happening, and how that benefits their own district. We don't have to convince our local congressman, he knows that very well. He's visited the lab a couple of times. And so they realise the economy of San Diego is influenced by it. But there are people everywhere, who are impacted by disease. And we can bring in patients along with us. And I can guarantee you, that the thing that moves people the most is when you walk in with a patient. And you have them meet the staff or the Congressperson that helps. And so that kind of thing. And stressing the idea that you don't get this unless you have the basic science. So taking care of basic science, from Thermus aquaticus to N -Acetylglucosamine is worthwhile, and it's going to help people in your district.
Rina Bogdanovic [01:02:48] Now I find it crazy. What direct contact can achieve.
Hudson Freeze [01:02:54] It makes all the difference in the world.
Rina Bogdanovic [01:02:56] I think a very good place for us to conclude is for me to ask you for advice for parents whose child might have been recently diagnosed with a Congenital Disorder of Glycosylation.
Hudson Freeze [01:03:09] Especially if you're in the US contact CDG Care. They're a really great organisation. And I think if it’s internationally, you know, then the World Conference is a good place to start. And you can have all of the connections you'll need there. They have resources for educating families. They have simple videos that educate parents and educate physicians about what CDG is. And we have special areas for professionals as well. So contact them. And the other one, of course, is the Frontiers of Congenital Disorders Glycosylation Consortium in the US, you get those on your list, reach out, and I can absolutely guarantee you, you're going to hear from people, probably sometimes within minutes, but it may take a day.
Rina Bogdanovic [01:04:11] Thank you very much for this conversation. What you're doing is incredible, I think just the journey you've taken from slime mould to the commitment you've had is really, really something to applaud. Thank you. Thank you for your persistence in this field.
Hudson Freeze [01:04:031] Well, thank you, Rina. I appreciate this opportunity to answer all the questions and the topics you brought up are the very ones if I had to go out and give a lecture, that's what I would talk about. And you've just set this up so perfectly. You're quite a good interviewer and I very much appreciate and keep up the great work. Okay.
Rina’s Outro
Rina Bogdanovic [01:04:52] Thank you very much. Now speaking to our listeners. I hope this conversation helped you better appreciate the integral role of glycosylation in normal physiological function, as well as the importance of collaboration between scientists, clinicians and patients. As Hudson said, we are all a part of the same ecosystem. If you would like to access more information about this conversation, Hudson's research, and links with useful materials for patients and professionals who want to learn more about CDG, follow the link in the description to the show notes for this episode. Equally, if you want to find out more about GlycanAge head on to glycanage.com where you can access a whole list of our scientific publications, blog posts, testimonials, and of course, this is where you can order your GlycanAge test kit. Watch out for our next episode, where I will be joined by Samia Mora, a cardiovascular health specialist whose research focuses on risk factors and the prevention of cardiovascular diseases. We'll be discussing cardiovascular disease risk factors associated with lifestyle and biological sex, as well as the potential use of IgG glycans as prognostic tools. Please don't forget to leave ratings and reviews for this episode, and engage with us on social media. Thank you for listening and have a great day.
Disclaimer
Please be advised that this show is for information only and should not be considered as a replacement or equal to medical advice.
