The Importance of IgG Glycosylation in Drug Development
IgG glycosylation is an important part of antibody-based drug production since it offers insights into the drug’s efficacy along with a more accurate prediction of the patient’s immune response to the drug.

Antibodies can be used preventatively as vaccines, but also as post-diagnosis treatment for many human diseases including cancer and immunologic illnesses. Both the production and market of therapeutic antibodies have undergone exponential growth in the past few years, and this trend is not expected to stop anytime soon (Lu et al. 2020).
A large portion of these therapeutic antibodies comprises of immunoglobulin G (IgG). IgG is a protein normally present in human plasma that is crucial to an efficient immune response against a variety of potential threats. The many functionalities of IgG include activation of complement components which can lead to a bactericidal effect, viral neutralization and toxin inactivation (Figure 1).
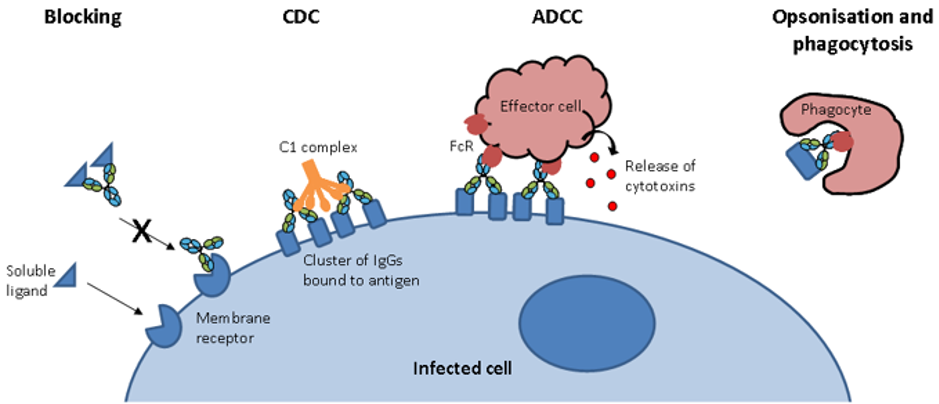
Figure 1. Antibody modes of action. Antibodies have several modes of action: i) block ligand-receptor interactions; ii) cause cell lysis through activation of complement dependant cytotoxicity (CDC); iii) interact with Fc receptors on effector cells to engage antibody-dependent cellular cytotoxicity (ADCC); iv) signal for ingestion of a pathogen by a phagocyte by opsonization.
The structure and function of IgG are vastly impacted by its glycosylation, more specifically by a conserved N-glycan attached to a single amino acid in the Fc domain of IgG. The composition of this N-glycan has the potential to significantly modify the function of IgG, even going so far as to switching it between anti-inflammatory and pro-inflammatory. Changes in Fc glycans modify the structural integrity of the Fc domain of IgG, thus impacting its affinity to Fcγ receptors. Since a considerable portion of IgG functions depend on its Fc domain binding to Fcγ receptors and the subsequent signalling, it is unsurprising that these glycan-induced changes in binding affinity cause considerable functional differences (Nose and Wigzell 1983). These notions are backed by the fact that nonpathogenic autoantibodies have been made pathogenic through selective isolation of IgG whose glycans did not contain galactose (Rademacher et al. 1994). Further proof of IgG glycan importance lies in the fact that reversely to the previous example, pathogenic antibodies have been made nonpathogenic by almost completely removing N-glycans (Nandakumar et al. 2007). The effect has even been confirmed in vivo on mice (Albert et al. 2008). There are many more cases in which IgG glycosylation has been correlated with a plethora of different human physiological states (Cobb 2019).
Knowing what we currently know about the effects of differential IgG glycosylation on its function, we can conclude that a deep understanding of IgG glycosylation is an important part of antibody-based drug production since it offers insights into the drug’s efficacy along with a more accurate prediction of the patient’s immune response to the drug. Moreover, this knowledge opens the theme of glycoengineering – is it possible that we will have therapeutic antibodies with glycans precisely modified in a way that would best serve the patient? Only time will tell, and judging by the rapidity of the growth of this field, we might not have to wait too long to find out.