Sialylation of Immunoglobulin E Is a Determinant of Allergic Pathogenicity
IgE glycan composition and specifically sialylation is established as an important regulator of allergic disease.

Immunoglobulin E (IgE) is a key molecule in allergies - the over-reaction of our immune system to non-harmful antigens from our surroundings. Upon sensitization, which happens at initial contact with antigen (in this case – allergen), allergen-specific IgE is synthesized and sits on the surface of cells (mastocytes and basophils) bound to a high-affinity receptor FcεRI. Following the next exposure, the allergen binds to these IgE molecules, crosslinking the FcεRI on the cell surface and triggering the immune cascade which results in a well-known spectrum of symptoms, ranging from mild runny nose and itching to life-threatening anaphylactic shock.
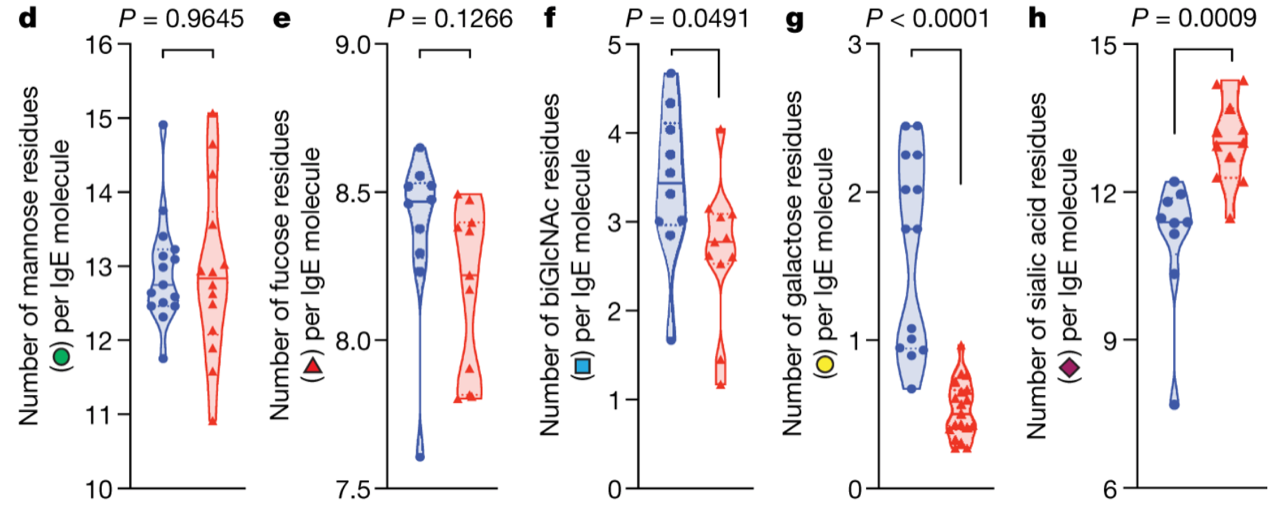
Total and allergen-specific IgE concentration in serum is routinely used to assess patients’ sensitization status. However, these measurements don’t accurately correlate with an allergic response. In the new Nature paper by the Anthony group at Harvard, we learn that the secret is in IgE glycans: IgE glycan composition and specifically sialylation is established as an important regulator of allergic disease.