A novel human coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in Wuhan, is currently one of the major health problems in the world. SARS-CoV-2 causes a respiratory illness named Coronavirus disease (COVID-19) and so far, we have 1 276 732 reported cases of COVID-19 and 56,985 COVID-19 related deaths confirmed by the World Health Organization (WHO). Currently, there are no specific vaccines or treatments for COVID-19. However, there are many ongoing clinical trials evaluating potential treatments.
Coronaviruses (CoVs) are the largest group of viruses belonging to the Nidovirales order, in which all the viruses are enveloped, non-segmented positive-sense RNA viruses. CoVs virus particles contain four main structural proteins. These are the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins, all of which are encoded within the 3′ end of the viral genome (Fehr and Perlman, 2015).
The S protein (∼150 kDa), utilizes an N-terminal signal sequence to gain access to the ER, and is heavily N-glycosylated (Fehr and Perlman, 2015). The trimeric S glycoprotein is a class I fusion protein and mediates attachment to the host receptor (Walls et al., 2020). In most, but not all coronaviruses, S protein is cleaved by a host cell furin-like protease into two separate polypeptides, noted S1 and S2. The S1 subunit facilitates the attachment of the virus, while the S2 subunit allows for the fusion of the viral and human cellular membranes. Human angiotensin-converting enzyme 2 (hACE2) is the entry receptor for SARS-CoV-2. Considering everything, the S protein is one of the major targets for the development of new vaccines (Shajahan et al., 2020).
As mentioned before, the S protein is highly glycosylated, with 22 predicted N-linked glycosylation sites a and four O-glycosylation sites on the S1 and S2 subunits. N-glycans play an important role in proper protein folding and priming by host proteases, including the S protein. Also, glycosylation can enable the coronavirus to evade immune responses by protecting amino acid residues from antibody recognition (Walls et al., 2020). Therefore, resolving glycosylation of the S protein may help in understanding viral binding with receptors, fusion, entry and replication. Furthermore, elucidating the glycosylation is an important step in designing suitable antigens for vaccine development
Shajahan et al. reported the site-specific quantitative N-linked and O-linked glycan profiling on SARS-CoV-2 subunits S1 and S2 through glycoproteomics using high-resolution LC-MS/MS. They identified the glycan composition at 17 out of the 22 predicted N-glycosylation sites of the SARS-CoV-2 S1 and S2 proteins and observed both high mannose and complex-type glycans but found no hybrid type N-glycans. They found predominantly highly processed sialylated complex-type glycans, which are at the receptor-binding domain (RBD) and can act as determinant in viral binding with hACE2 receptors.
Furthermore, they observed two unexpected O-glycosylation modifications on the RBD of spike protein subunit S1. Even though O-glycosylation has been predicted on the spike protein of SARS-Cov-2, this is the first time that experimental data for the exact O-glycosylation site and identification of O-glycans attached on the subunit S1 have been reported. Although it is unclear what is the function of these predicted O-linked glycans, the proposed theory is that they create a "mucin-like domain" that could shield SARS-CoV-2 spike protein epitope. Since some viruses can utilize mucin-like domains as a glycan shield for immunoevasion, determination of predicted O-linked glycosylation sites is increasingly being investigated.
These data suggest that detailed glycan analysis and potential post-translational modifications of the SARS-CoV-2 spike protein is very important for the development of glycoprotein-based vaccine. Also, understanding of complex sialylated N-glycans and sialylated mucin-type O-glycansis useful for understanding the pathology of virus binding and the infection itself in future therapeutic possibilities.
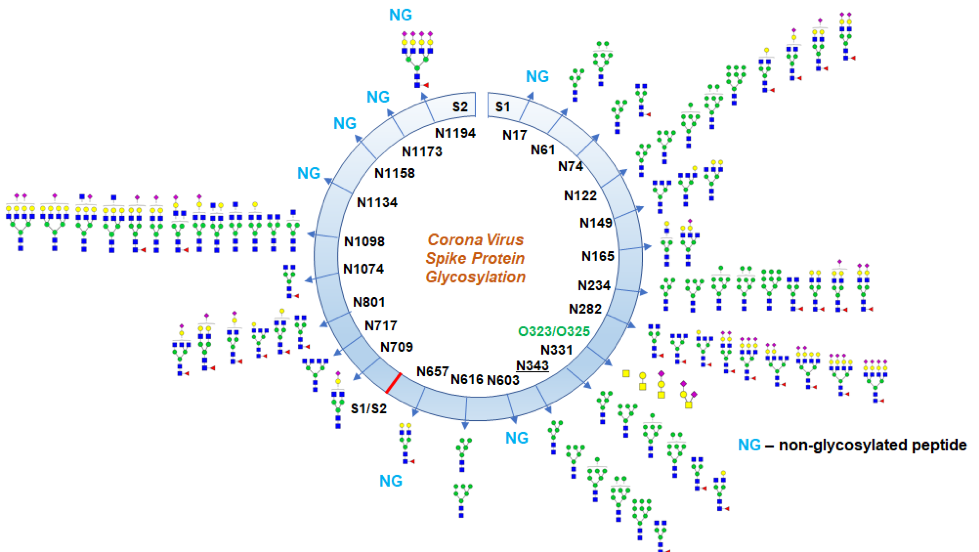
Glycosylation profile on coronavirus SARS-CoV-2 characterized by high-resolution LC-MS/MS (from Shajahan et al., bioRxiv, 2020)
Other articles you may like:
 Glycoscience
GlycoscienceUnderstanding Glycans in COVID-19 Drug Design
We need to intensify studies on the role of glycosylation and understand the importance of these complicated structures if we want to have success in the diagnosis, treatment, and prevention of the COVID-19.
Read full article  Glycoscience
GlycoscienceGlycans in Drug Discovery - HIV Glycopeptide Antigens
Glycoproteins found at the surface of several viruses are called viral envelope glycoproteins and they are great targets for virus neutralization.
Read full article  Glycoscience
Glycoscience.jpg?alt=media&token=b563cc51-2d7e-4da5-ba32-4b1bcc01fae3)


