Each virus possesses a specific entry protein that acts as a key, unlocking the gates to the host cells. This viral entry protein usually binds to one of the host proteins, “the lock”, and such interactions are crucial for the infection and propagation of the virus [1]. Coronaviruses, in particular, are notoriously versatile in terms of which host proteins they can recognize, and this is thought to have helped them to spread among almost all mammal and bird species [2]. Currently, humanity has been hit by one of the most serious pandemics in decades, caused by one of the coronaviruses, named SARS-CoV-2, that inflicts a severe respiratory disease on some people. To be able to stop the disease, it is of vital importance to understand how this virus invades human cells and what makes some people particularly susceptible to SARS-CoV-2 infection.
The more efficiently the virus recognizes the host cell receptors, the stronger is its power to infect new individuals and transmit in the population. It has already been established, that the spike protein of SARS-CoV-2 primarily recognizes angiotensin-converting enzyme 2 (ACE2)[3], which is a glycoprotein found on the surface of endothelial cells in the lungs, intestines, heart, arteries and kidneys. However, ACE2 is not the only factor that influences viral entry. For instance, plenty of human viruses recognize host cell glycosylation motives as additional attachment factors [4,5], coronaviruses being no exception[2]. Recently, Hao et al. showed [6] that a special type of glycans, heparan sulfates (HS), abundant on the outer cell surfaces, can aid the SARS-CoV-2 virus binding to target cells. HSs are linear chains of sugar residues attached to proteins, rich in uronic acids and extensively sulfated. Differences in length, composition and abundance create a great diversity of HS structures and their strong negative charge makes them an attractive target for viral attachment [4]. Hao et al. observed stronger binding of the viral spike to HS oligosaccharides with higher sulfation degree, strongly preferring those with 6-O-sulfate groups. Hao et al also created a model structure of the SARS-CoV-2 spike protein in complex with HS. According to their model, initial binding of HSs may change the 3D conformation of the spike in such a way that it will be more likely to interact with the ACE2 receptor and, finally, enter the host cell (Fig. 1).
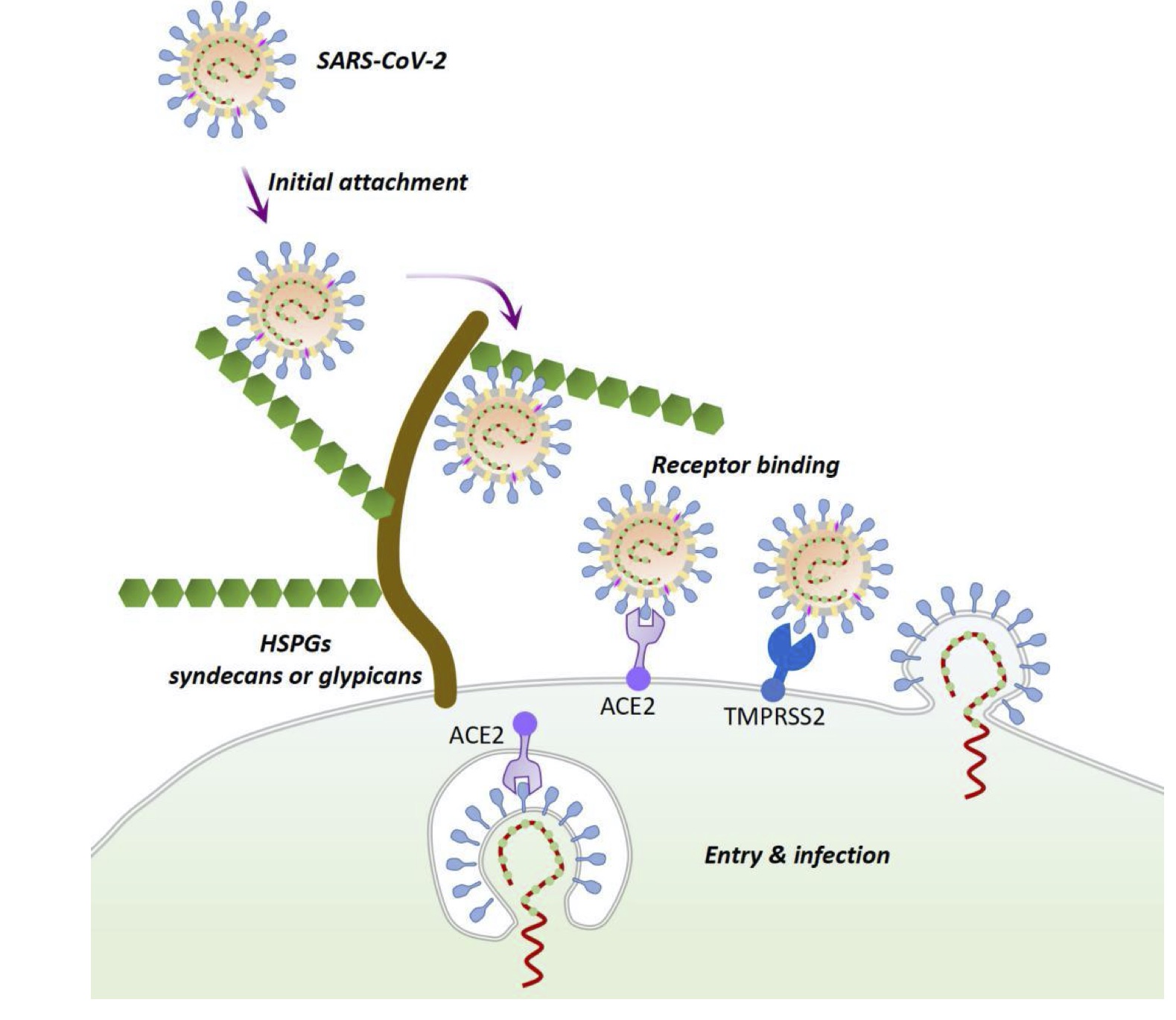
Fig 1. A possible mechanism for SARS-CoV-2 entry and infection through interaction with heparan sulfate proteoglycans [6].
Not only host glycans can influence viral infection. When speaking of SARS-CoV-2, one should bear in mind, that the lungs and upper respiratory tract, target organs of the virus, are home to various bacterial microorganisms, whose capsids are covered by a rich variety of polysaccharides and lipopolysaccharides. These glycans might as well modulate the binding of the virus to the tissues. Chiodo et al. [7] discovered that some of the Streptococcus pneumonia and Pseudomonas aeruginosa capsid polysaccharides that contain rhamnose, a sugar residue, that is not found in humans, indeed interact with the viral spike protein, as opposed to the glycans of other bacteria that are usually not a part of the lung microbiome. Thus, SARS-CoV-2 seems to use a helping hand of the local microbiota to efficiently bind and infect the epithelial cells of the respiratory tract.
Finally, let us not forget that the spike protein of the SARS-CoV-2 virus is itself extensively glycosylated [8]. These glycans, which are not encoded in the viral genome but synthesized by the host glycosylation machinery, can be recognized by numerous lectins, carbohydrate-binding proteins, that are very abundant on the immune cells. In this manner the viral glycome can modulate the host immune response, either facilitating or inhibiting viral propagation in the host. For instance, Chiodo et al. [7] observed SARS-CoV-2 spike binding to DC-SIGN and MGL lectins, both of which can be found on macrophages and dendritic cells that induce immune tolerance. Some members of the Siglec family of lectins that recognize α2,6 and α2,3 linked sialic acids also were shown to interact with the viral spike protein. Siglecs are expressed on many immune cell types, is an important part of signal transmission during an immune response, as well as common target for viruses [4].
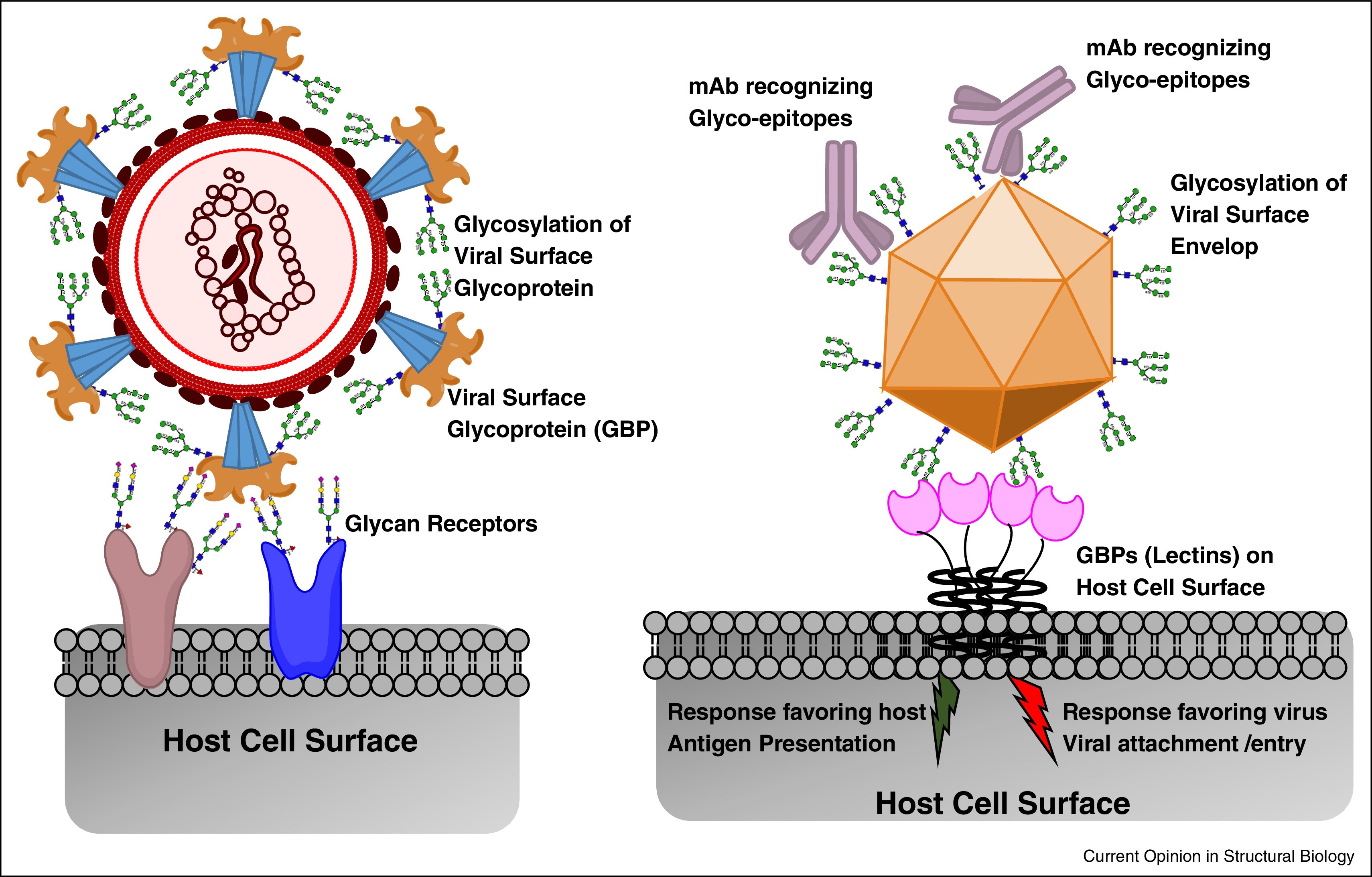
Fig. 2. Role of complex glycans in the interplay between the pathogen and host [4]
Thus, it has already become obvious that glycans play an important role in the course of SARS-CoV-2 infection and should not be overlooked when searching for ways to understand and control this new disease. Glycans might become a great biomarker tool to predict disease severity, response to therapy and recovery prognosis. Current research suggests that SARS-CoV-2 shows marked preference towards particular glycan structures and lectins, which are either potential entry co-receptors or modulators of the immune response (Fig. 2). Since every human being has its unique glycan and microbiome “fingerprint”, we can expect to find some people who are protected from the novel virus by their own or bacterial glycans, as well as those, whom their glycomes put at a higher risk of developing severe symptoms.
1. Maginnis MS. Virus–Receptor Interactions: The Key to Cellular Invasion. Journal of Molecular Biology. Academic Press; 2018. pp. 2590–2611. doi:10.1016/j.jmb.2018.06.024
2. Forni D, Cagliani R, Clerici M, Sironi M. Molecular Evolution of Human Coronavirus Genomes. Trends in Microbiology. Elsevier Ltd; 2017. pp. 35–48. doi:10.1016/j.tim.2016.09.001
3. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181: 281-292.e6. doi:10.1016/j.cell.2020.02.058
4. Raman R, Tharakaraman K, Sasisekharan V, Sasisekharan R. Glycan–protein interactions in viral pathogenesis. Current Opinion in Structural Biology. Elsevier Ltd; 2016. pp. 153–162. doi:10.1016/j.sbi.2016.10.003
5. Kreisman LS, Cobb BA. Infection, inflammation and host carbohydrates: A Glyco-Evasion Hypothesis. Glycobiology. 2012;22: 1019–1030. doi:10.1093/glycob/cws070
6. Hao W, Ma B, Li Z, Wang X, Gao X, Li Y, et al. Binding of the SARS-CoV-2 Spike Protein to Glycans. bioRxiv. 2020; 2020.05.17.100537. doi:10.1101/2020.05.17.100537
7. Chiodo F, Bruijns SCM, Rodriguez E, Li RJE, Molinaro A, Silipo A, et al. Novel ACE2-Independent Carbohydrate-Binding of SARS-CoV-2 Spike Protein to Host Lectins and Lung Microbiota. bioRxiv. 2020; 2020.05.13.092478. doi:10.1101/2020.05.13.092478
8. Shajahan A, Supekar NT, Gleinich AS, Azadi P. Deducing the N- and O- glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. biorxiv.org. doi:10.1101/2020.04.01.020966
 Health
Health